What Term Best Describes a Reaction That Releases Energy
The free energy of the system decreases. Chemical equilibrium is relatively rare in.
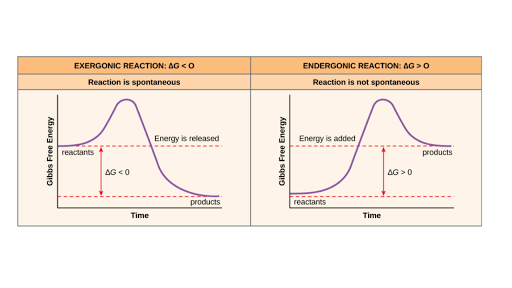
Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
The reactions are nonspontaneous.
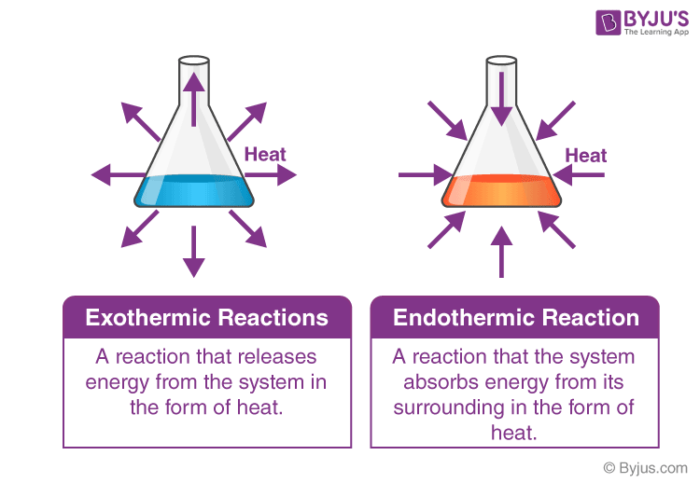
. Finally What is the energy released in a reaction Energy and Chemical Reactions These reactions called exothermic reactions release energy. Today were doing problem for which of the following reactions releases energy and were given for choices because this is a multiple choice practice question for the AP biology exam. Some reactants will be converted to products.
1 What Is The Term Used To Describe The Energy Needed To Get A Reaction Started. A substance that affects the reaction rate without being used up in the reaction Decomposition A reaction in which a compound breaks down into two or more simpler substances. The chemical bonds formed from the reaction are stronger than those that were broken in the reactants.
Chemical Reactions and Energy. 4 chemical and energy is absorbed. I understand what the basic energy source for all biological processes and that is probably heard of it.
Which of the following terms best describes the forward reaction in the figure. An exergonic reaction is a reaction that releases free energy. A net input of energy from the surroundings is required for the reactions to proceed.
A The products have more total energy than the reactants. BEndergonic reactions consume energy and exergonic reactions release energy. Decreases the activation energy.
What does it mean for a reaction to release energy1 point The relative potential energy of the reaction is positive. C The reaction goes only in a forward direction. Energy that drives the attachment of a nucleotide to the end of a growing st 000 What reaction will release the largest amount of energy to help power anothe.
The activation energy of the reaction is positive. We first have to. N 92235U 52137Te 4097Zr 2n.
Because this type of reaction releases energy rather than consuming it it can occur spontaneously without being forced by outside factors. The relative potential energy of the reaction is negative. This is nuclear reaction because nucleus of an atom in thus example uranium-235 and a subatomic particle neutron from outside the atom collide and produce two nuclides tellurium-137 and zirconium-97 that are different from the nuclide that.
4 Why is activation energy required to start some reactions. 3 What is the term for the amount of energy that needs to be added for a chemical reaction to start quizlet. DEndergonic reactions take place slowly and exergonic reactions take place quickly.
Releases more energy than it absorbs. The activation energy of the reaction is positive. Gluconeogenesis is an anabolic process because it involves building glucose molecules through endergonic reactions that require the input of energy ATP to work.
What is the term for the amount of energy that needs to be added for a chemical reaction to start. The relative potential energy of the reaction is positive. B The reaction proceeds with a net release of free energy.
2 What term is used to describe the energy required to start a reaction. The products have more total energy than the reactants. Which statement best describes the graph shown.
OOOO the energy a compound possesses by virtue of its chemical bonds and their orientation the energy required to remove an electron from a gaseous atom the amount of energy absorbed or released when a chemical reaction takes place the energy required to separate the ions in a crystalline solid the minimum net kinetic energy that colliding particles must possess in order. All reactants will be converted to products but no products will be converted to reactants. An exergonic reaction may be called a spontaneous reaction or a favorable reaction.
Enzyme-catalyzed reactions release more free energy than noncatalyzed reactions The following questions are based on the reaction AB - CD Free Energy C D Progress of the Reaction 8. Which reaction releases the greatest amount of energy per 2 moles of product. Okay so its understand this question.
Exothermic reactions are reactions or processes that release energy usually in the form of heat or light. The reaction proceeds with a net release of free energy. Furthermore What is the term used to describe the energy needed to get a reaction started group of answer choices Activation energy is the term used to describe the energy needed to get a reaction started.
Which of the following terms describes a reaction in which there is a net transfer of energy from a system to its surroundings - that is where more energy is released by bond formation than is consumed by bond cleavage. Which phrase best describes the effect of a catalyst on a chemical reaction. Exergonic reactions release energy to the surroundings.
What best describes the reaction It is an anabolic process with endergonic reactions that require an input of energy. D A net input of energy from the surroundings is required for the reactions to proceed. CBoth endergonic and exergonic reactions require a small amount of energy to overcome an activation barrier.
Enzyme-catalyzed reactions require energy to activate the enzyme e. Which statement best describes a chemical reaction in which energy is released. In an exothermic reaction energy is released because the total energy of the products is less than the total energy of the reactants.
In chemistry terms exergonic reactions are reactions where the change in free energy is negative.
How Is Exothermic And Endothermic Reaction Connected To Change In Energy Quora
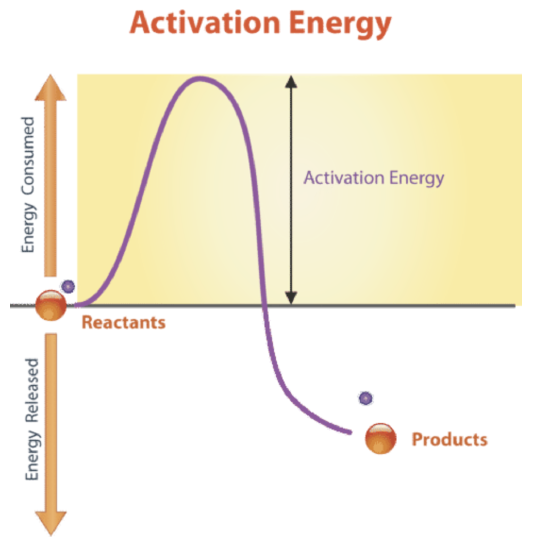
3 9 Energy In Chemical Reactions Biology Libretexts
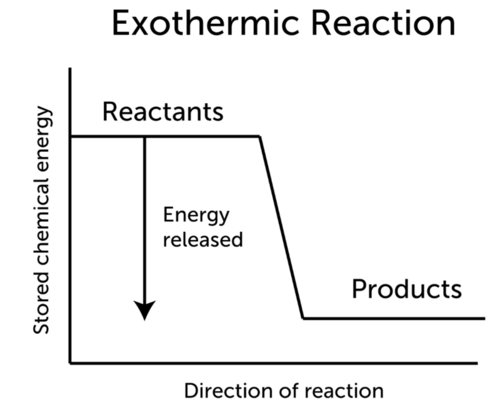
Exothermic Reaction Ck 12 Foundation

Energy Changes In Chemical Reactions Introduction To Chemistry

Difference Between Endothermic And Exothermic Reactions Chemistry

Based On The Information In The Graph Choose The Statement That Best Describes The Reaction It Brainly Com
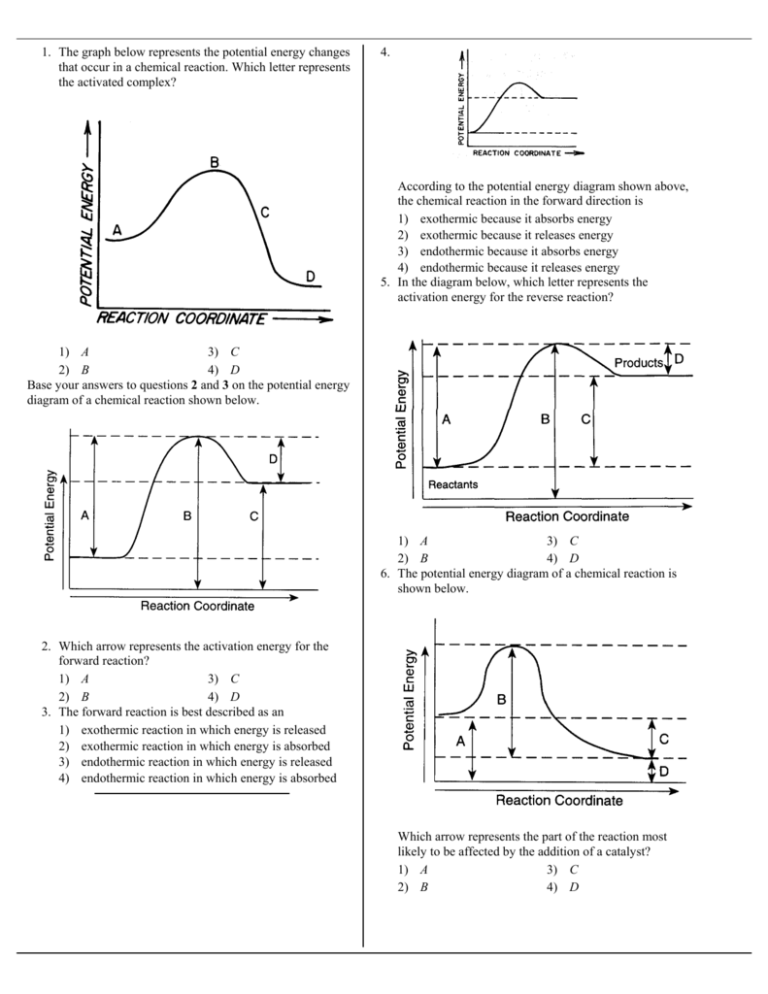
1 The Graph Below Represents The Potential Energy
In Chemical Reactions What Is The Role Of Energy Quora

Exothermic And Endothermic Processes Chemistry For Non Majors

Exothermic And Endothermic Processes Introduction To Chemistry
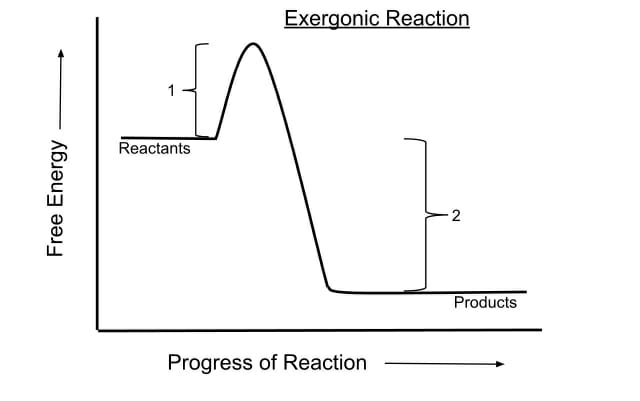
Exergonic Reaction Definition Examples And Quiz Biology Dictionary
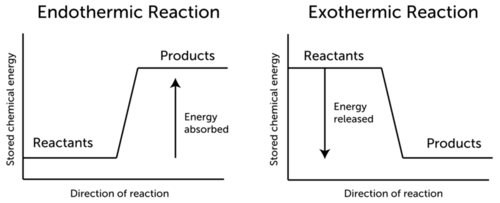
Conservation Of Energy In Chemical Reactions Ck 12 Foundation
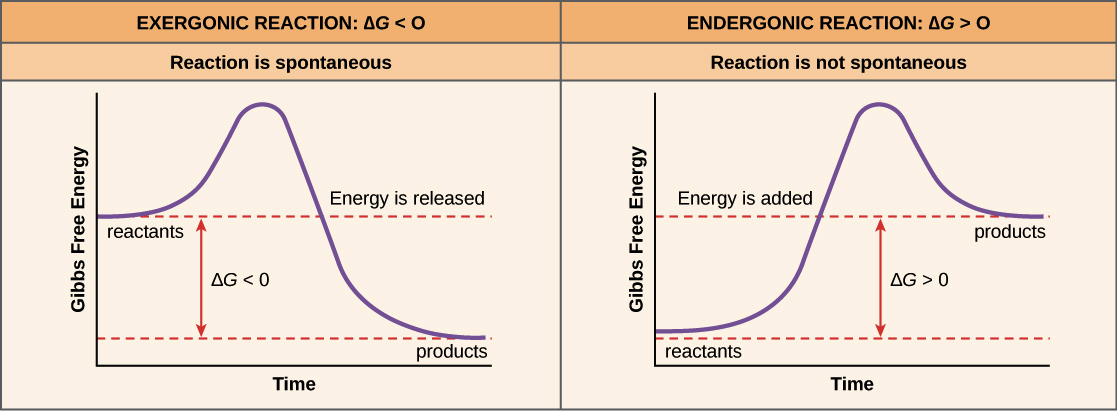
Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

Endothermic And Exothermic Reactions Ppt Video Online Download

Exothermic And Endothermic Processes Introduction To Chemistry
Why Do Exothermic Reactions Require Activation Energy Quora

Endothermic And Exothermic Reactions Ppt Video Online Download


Comments
Post a Comment